Let the particles do the work, part 2
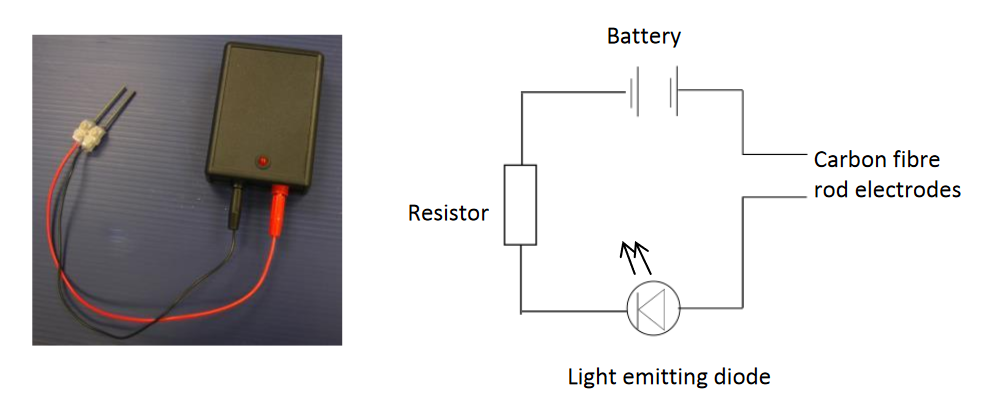
CLEAPSS devised a simple small electrical conductivity indicator. The resistor in the circuit limits the current to protect the Light Emitting Diode (LED). The carbon fibre-rod electrodes are very strong unlike graphite.
CLEAPSS devised a simple small electrical conductivity indicator. The resistor in the circuit limits the current to protect the Light Emitting Diode (LED). The carbon fibre-rod electrodes are very strong unlike graphite.
It was originally designed for our primary section at CLEAPSS who thought it might be useful for straightforward investigation of what conducts electricity what does not. However, this little piece of kit has grown in value in my estimation over the past years because it has given me a greater insight to the nano world where all the chemical action takes place.
Its use in secondary education though appears limited to teachers because that concept of electrical conduction applying to metals and non-metals carries on the students to 16 and beyond. The addition is that molten salts and aqueous salt solutions conduct electricity.
In one of our specifications for chemistry for students to 16, the word conductivity comes up 7 times always in relation to metals and non-metals. The word “electrolysis” appears 27 times. The macro event of seeing metals and gas bubbling at electrodes, has over taken the nano interpretation. Electrolysis (chemical reactions at the positive and negative electrodes) occurs to enable the conductivity electrical current to pass through a liquid (no wires).
If the electrodes are placed in ethanol or cyclohexane, there is no light coming from the red LED on the conductivity indicator. If it is placed in pure water, there is no light but if placed in tap water, the light comes on. This happens with salt water but not water with refined sugar (sucrose) or glucose.
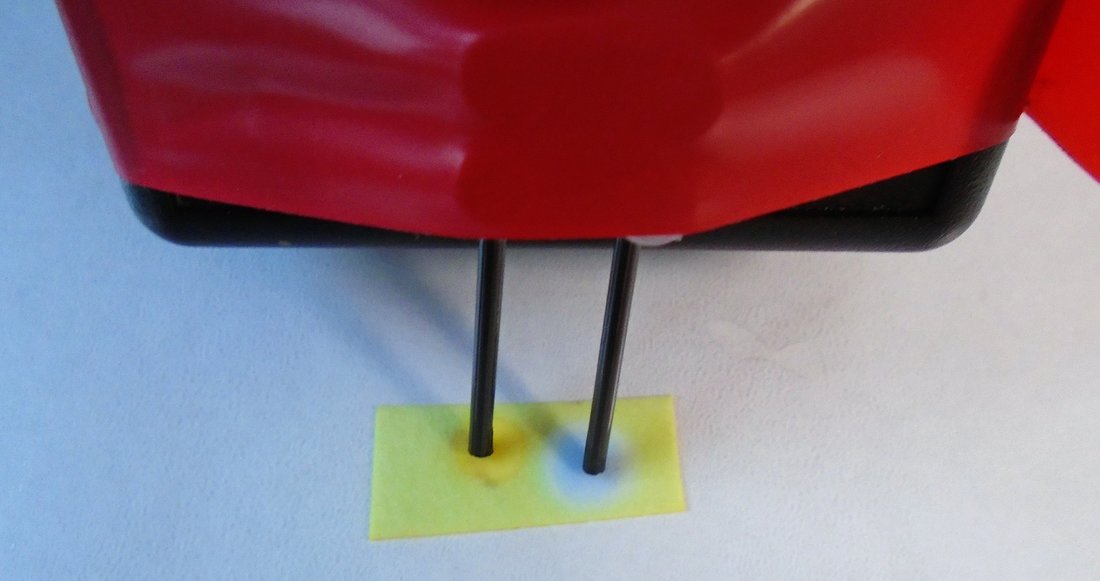
In the photos below, the red LED is not showing when the electrodes are in pure water. When solid potassium manganate(VII) is added to water between the 2 electrodes and the potassium manganate dissolves, the LED lights up BUT the purple colour moves to the (red) positive electrode.
In one of our specifications for chemistry for students to 16, the word conductivity comes up 7 times always in relation to metals and non-metals. The word “electrolysis” appears 27 times. The macro event of seeing metals and gas bubbling at electrodes, has over taken the nano interpretation. Electrolysis (chemical reactions at the positive and negative electrodes) occurs to enable the conductivity electrical current to pass through a liquid (no wires).
If the electrodes are placed in ethanol or cyclohexane, there is no light coming from the red LED on the conductivity indicator. If it is placed in pure water, there is no light but if placed in tap water, the light comes on. This happens with salt water but not water with refined sugar (sucrose) or glucose.
In the photos below, the red LED is not showing when the electrodes are in pure water. When solid potassium manganate(VII) is added to water between the 2 electrodes and the potassium manganate dissolves, the LED lights up BUT the purple colour moves to the (red) positive electrode.

When this is carried out in the puddle, containing dissolved potassium manganate(VII), and the electrode held for 30 seconds and then removed slowly, the areas where the electrodes have been show up. The purple colour has been repelled from the negative electrode and attracted to the positive electrode. Could we use this as evidence to imply that the purple particle carries a negative charge? I do not get an electric shock from potassium manganate(VII) as I would from an electrostatic charge, so there must be an accompanying positive charged present as well so that the solid is electrically neutral.
Could it be that what we thought was 1 purple particle in potassium manganate(VII) when potassium manganate(VII) dissolves in water, is possibly 2 or even more electrically charged particles, one of which is purple negatively charged and attracted to the positive electrode?
Here is a more a more dramatic demonstration. It does use a very toxic copper chromate. A risk analysis on the safety requires material is kept to the minimum, which is possible using a microscale technique. Chromates are banned from use in schools of many countries on toxic and environmental grounds. “As the dose makes the poison”, this is so minimalist, and the educational important so important, I really think that it should be carried as a demonstration at least with the result projected onto the screen.
One drop of 0.1M copper sulfate solution (blue) is added to 1 drop of 0.1M potassium chromate solution (yellow). A Brown solid appears. Now add 2 to 3 drops of 2M ammonia and a green solution (blue and yellow combined colours) appears.
About 2 drops of this green solution are placed on filter paper and the electrodes applied for 60 seconds. The blue copper particle is attracted to the negative electrode and yellow chromate particle is attracted to the positive electrode. (Chemists will realise that I am emphasising the blue colour by using ammonia solution which forms the deeper blue Cu(NH3)4 dipostive ion.)
Could it be that what we thought was 1 purple particle in potassium manganate(VII) when potassium manganate(VII) dissolves in water, is possibly 2 or even more electrically charged particles, one of which is purple negatively charged and attracted to the positive electrode?
Here is a more a more dramatic demonstration. It does use a very toxic copper chromate. A risk analysis on the safety requires material is kept to the minimum, which is possible using a microscale technique. Chromates are banned from use in schools of many countries on toxic and environmental grounds. “As the dose makes the poison”, this is so minimalist, and the educational important so important, I really think that it should be carried as a demonstration at least with the result projected onto the screen.
One drop of 0.1M copper sulfate solution (blue) is added to 1 drop of 0.1M potassium chromate solution (yellow). A Brown solid appears. Now add 2 to 3 drops of 2M ammonia and a green solution (blue and yellow combined colours) appears.
About 2 drops of this green solution are placed on filter paper and the electrodes applied for 60 seconds. The blue copper particle is attracted to the negative electrode and yellow chromate particle is attracted to the positive electrode. (Chemists will realise that I am emphasising the blue colour by using ammonia solution which forms the deeper blue Cu(NH3)4 dipostive ion.)
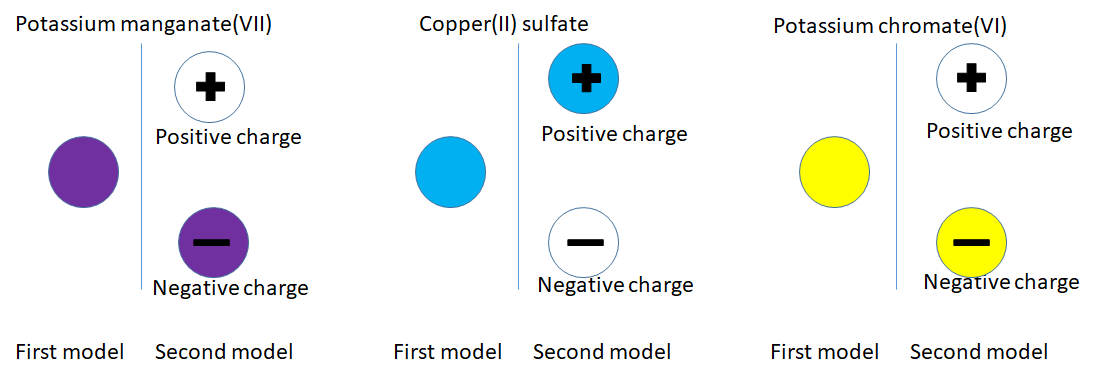
The blue particles are positively charged because they are attracted to the negative electrode. The yellow particles are negatively charged because they are attracted to the positive electrode. I think we need to improve the particle model.
Going back to the potassium manganate(VII) particle, what was a purple particle appears to be comprised of a purple negative particle and colourless positive particle.
What we thought was a blue copper sulfate(blue) and potassium chromate(yellow) particle is more complicated as shown in the diagrams below.
Going back to the potassium manganate(VII) particle, what was a purple particle appears to be comprised of a purple negative particle and colourless positive particle.
What we thought was a blue copper sulfate(blue) and potassium chromate(yellow) particle is more complicated as shown in the diagrams below.
I have tried to develop this argument in the spirit of Alex Johnstone. Start with what students know and what students can see.
When it comes to salt, which students know is a colourless solid, dissolves in water (as in sea water) and also conducts an electric current, we have to say there is not one particles of sodium chloride but 2 particles, one negative and one positive. (Purists will say that the copper, sulfate and chromate ions carry two charges. Yes but one thing at time, otherwise we shall have cognitive overload.
Students can carry out an investigation on any salt. Some will just light up the LED and bubbles appear around the electrodes. Others will start to produce metals at the positive electrode and halogens around the positive electrodes. This video shows the addition of a tiny sample of tin chloride, running at 4 times the normal speed.
When it comes to salt, which students know is a colourless solid, dissolves in water (as in sea water) and also conducts an electric current, we have to say there is not one particles of sodium chloride but 2 particles, one negative and one positive. (Purists will say that the copper, sulfate and chromate ions carry two charges. Yes but one thing at time, otherwise we shall have cognitive overload.
Students can carry out an investigation on any salt. Some will just light up the LED and bubbles appear around the electrodes. Others will start to produce metals at the positive electrode and halogens around the positive electrodes. This video shows the addition of a tiny sample of tin chloride, running at 4 times the normal speed.
More spectacular is the addition of a neodymium magnet below the puddle. Here is the addition of potassium manganate(VII) to the puddle. The positive electrode is on the right and the S-pole is facing upwards and situated underneath the Petri dish. Now I am having trouble with the explanation at the nano level. Next blog will be the role of water in all chemistry. I think this is developing into a “reader” for school students. All the textbooks in the UK are aimed at revision for an exam but none study the holistic nature of the subject.