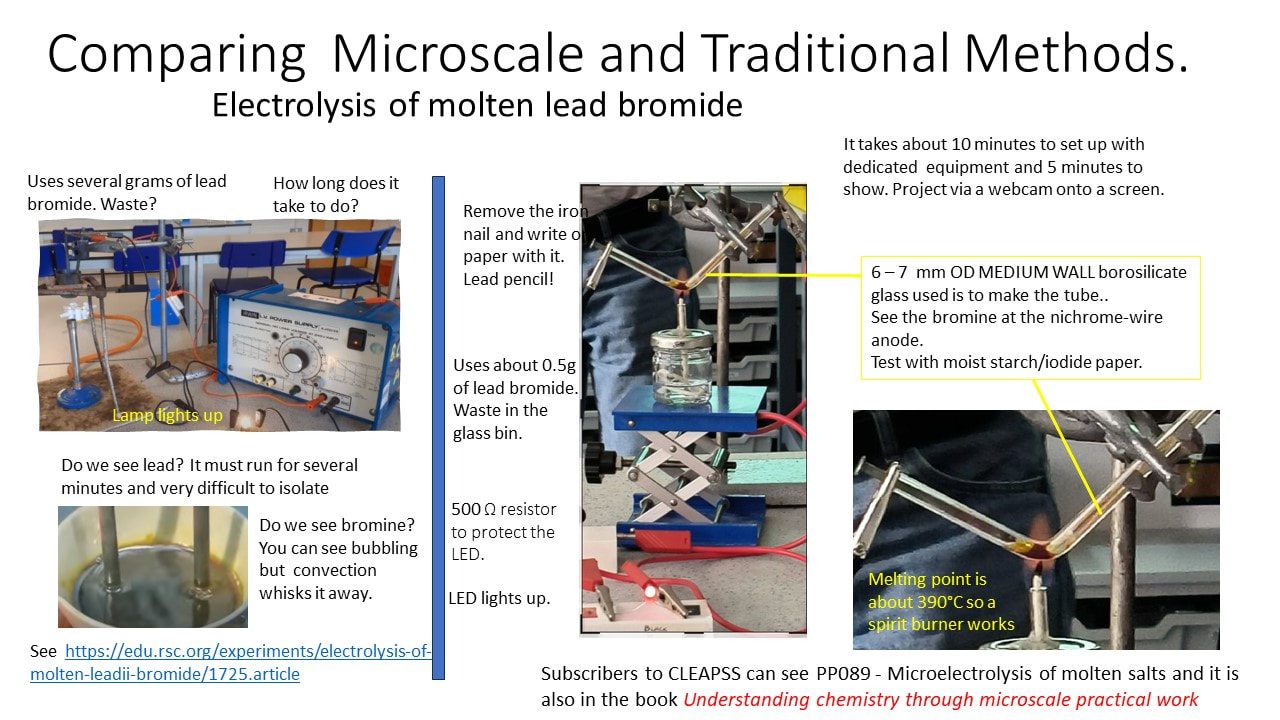
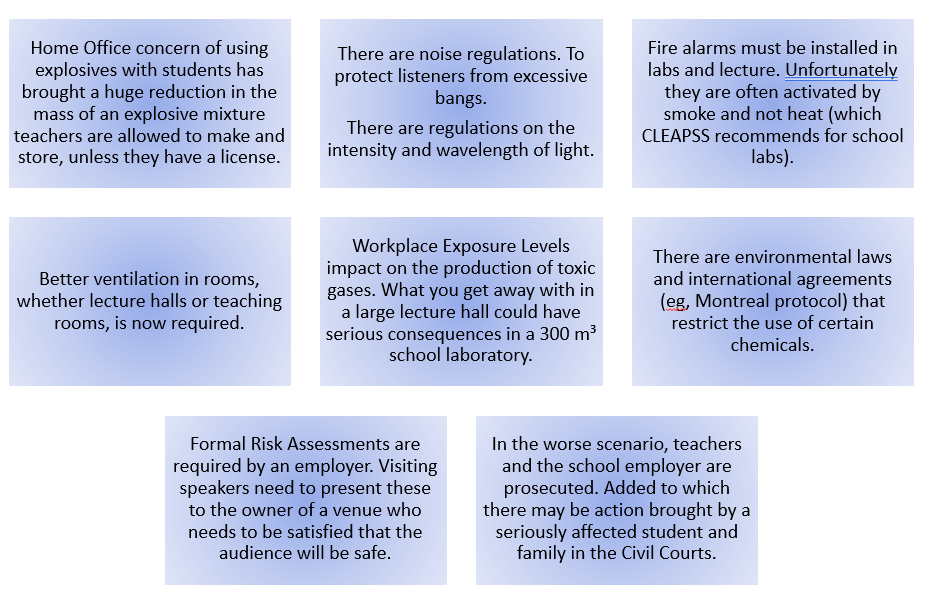
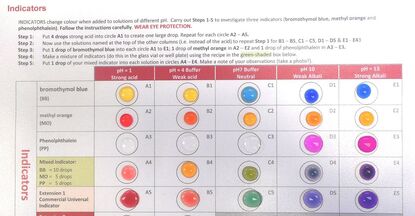
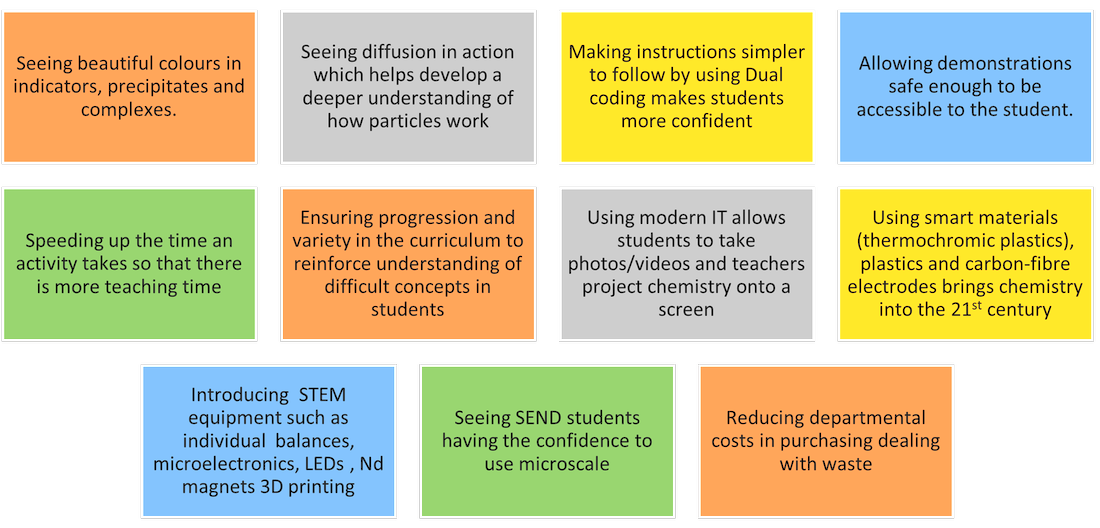
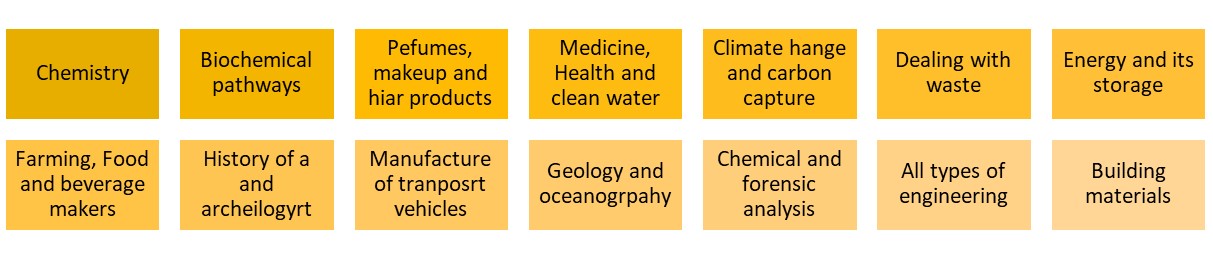
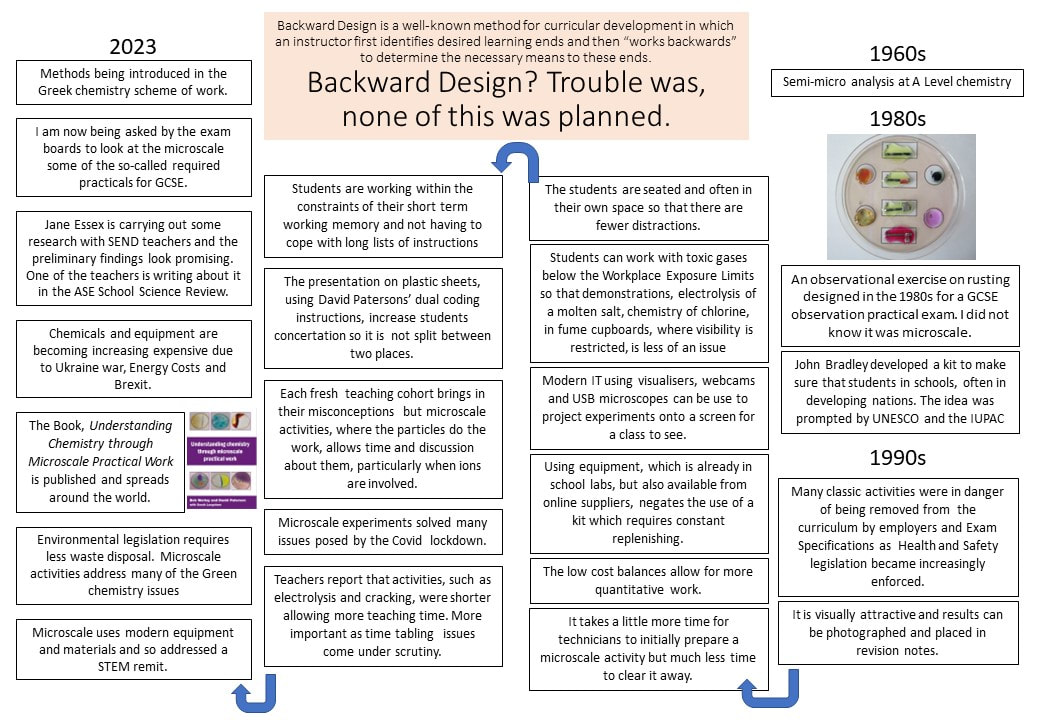
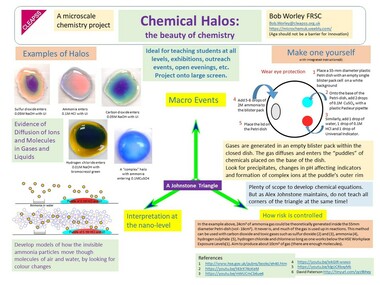
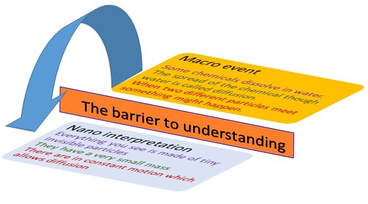
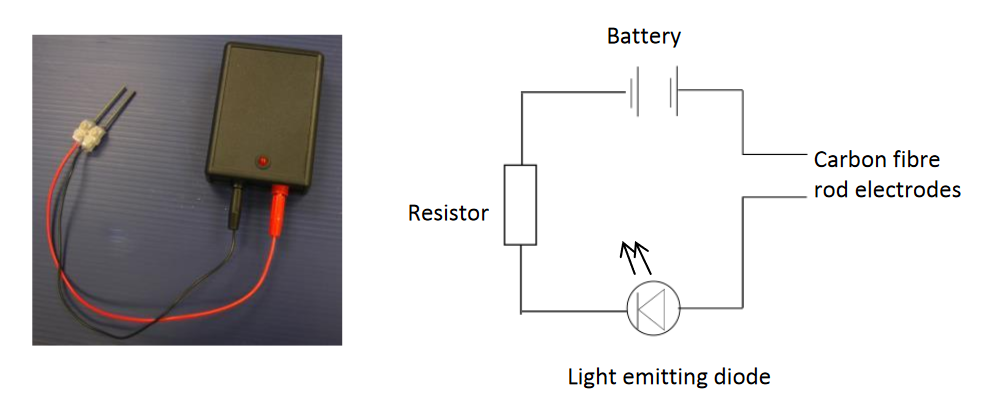
I had a helpline recently from a school technician who suggested my microscale method, which he had set up in a previous school, to his new teachers. But no! Teachers said this microscale method is not dramatic enough (had they ever tried it?), even though it provides better chemical evidence to improve students’ knowledge. Is a chemical demonstration for knowledge/teaching/learning or drama? The equipment for both is shown in the visual presentation.
- you see LED light up.
- you can remove the iron nail from the melt and on cooling make a few marks of lead on paper - a lead pencil
- you see the red bromine vapour and can test it with starch iodide paper.
- it can be set up quickly, and does not require a fume cupboard,
- it uses minimal amounts of lead bromide (0.5 to 0.7 g), which is toxic, and large amounts need careful waste management, and
- it can be projected using webcams, onto a screen.
There is a perception amongst some teachers that microchemistry is not real chemistry because it was not how they were taught. They are also concerned that students would be at a disadvantage in exams, although there is no evidence of this. The ideas are there to complement the traditional demos and experiments and add variety to teaching with an alternative approach.
I am old enough to remember similar comments when Nuffield Chemistry was introduced in the late 1960s and 70s.
Still, a demonstration can be enjoyable if not a drama!